The CDC or Center for Disease Control and Prevention has issued an urgent warning against eye drops that are responsible for a rare bacterial infections.
The infection has wreaked havoc and resulted in an outbreak, hence why the CDC ordered a recall. This rare bacteria is not only resistant to antibiotics but has also led to a loss of vision, eyeballs, and even 3 deaths.
Read along as we divulge everything we know about the eye drops and bacteria responsible for this unexpected outbreak.
The CDC has warned of an outbreak

The rare infection has affected 68 people so far and is responsible for 3 deaths. The worst part however is that the infection is resistant to most antibiotics, therefore making it tougher for medical professionals to treat it. It has also posed a threat to patients’ lives and could result in them losing their vision.
The rare bacteria is linked to eye drops available over-the-counter
A rare bacteria called Pseudomonas aeruginosa has infected people across 16 US States. The outbreak has been connected to 8 recent reports of vision loss and four reports of enucleation, or surgical removal of an eyeball.
The infection is linked to certain artificial-tear products available over the counter and has resulted in the spread of the deadly bacterial infection.
The eye drops may have been contaminated after coming in contact with environmental agents during handling, experts believe. The bacterium is resistant to most antibiotics and is particularly dangerous in healthcare settings and for people with weak immune systems.

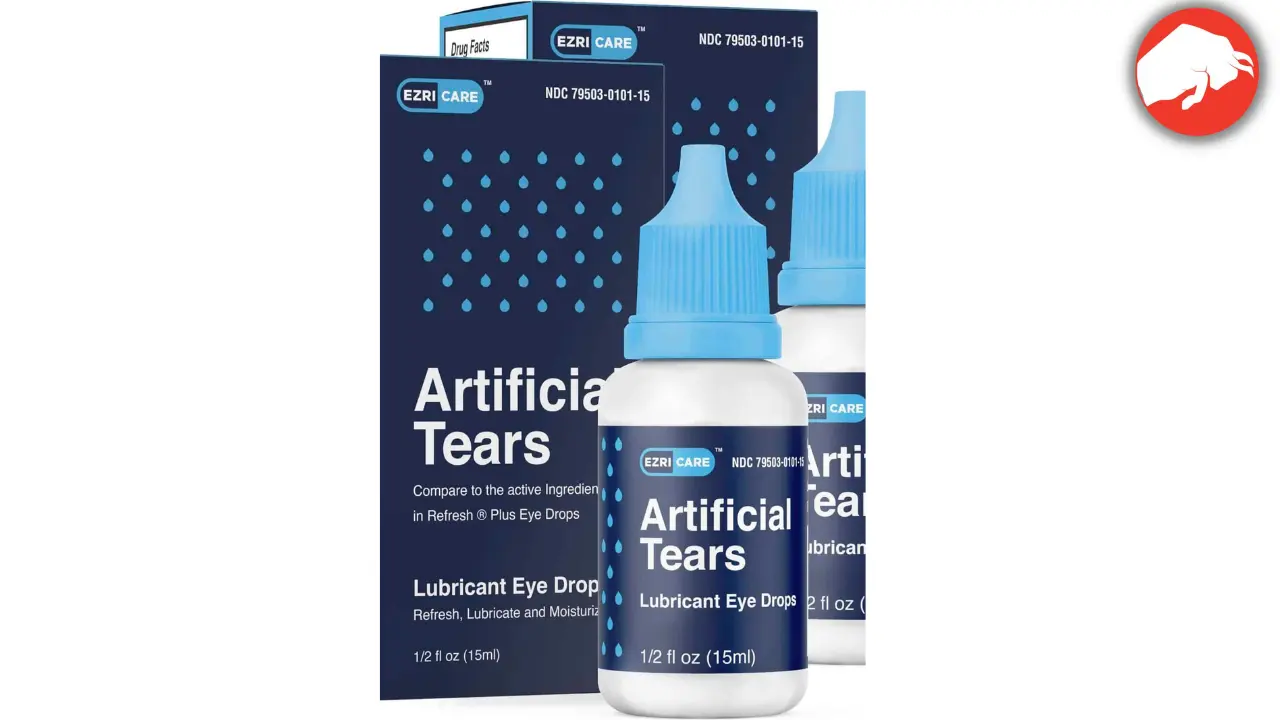
The CDC first issued a warning in January after a few cases were reported. Shortly after the warning, EzriCare Artificial Tears, a preservative-free, over-the-counter product that many infected patients reported using, was recalled by its manufacturer.
Since then, several other eye products have been taken off shelves, although not all have been linked to bacterial infection.
These eye drops have been recalled

At least 4 eye drops have been recalled so far. These include the aforementioned EzriCare and Delsam Pharma’s Artificial Tears, which have been associated with bacterial infection.
Two other products namely, Pharmedica USA’s Purely Soothing, 15% MSM Drops, and Apotex’s Brimonidine Tartrate Ophthalmic Solution, 0.15% have also been taken off-shelves by their manufacturers, though they are not linked to the infection.
Watch out for these symptoms
The CDC has listed symptoms you need to watch out for if you have used Ezricare and Delsam Pharma’s Artificial Tears.
The symptoms include yellow, green, or clear discharge from the eye; eye pain or discomfort; redness of the eye or eyelid; a sensation that there is something in your eye; increased sensitivity to light; and blurry vision,
The CDC also instructs those who have used the eye drops and experienced these symptoms to seek medical care immediately. People not experiencing symptoms do not need to test for possible infection, it says.
A severe eye infection can feel similar to less threatening conditions such as dry eye disease and other autoimmune or inflammatory diseases of the eyes, experts caution.