HIV/AIDS Cure 2019 reaches a new level. The disease is a dreadful epidemic. This is because the disease has no cure. Researchers are trying to develop a cure for it. They have come a long way thanks to the advancement in science and technology.
[fvplayer id=”339″]
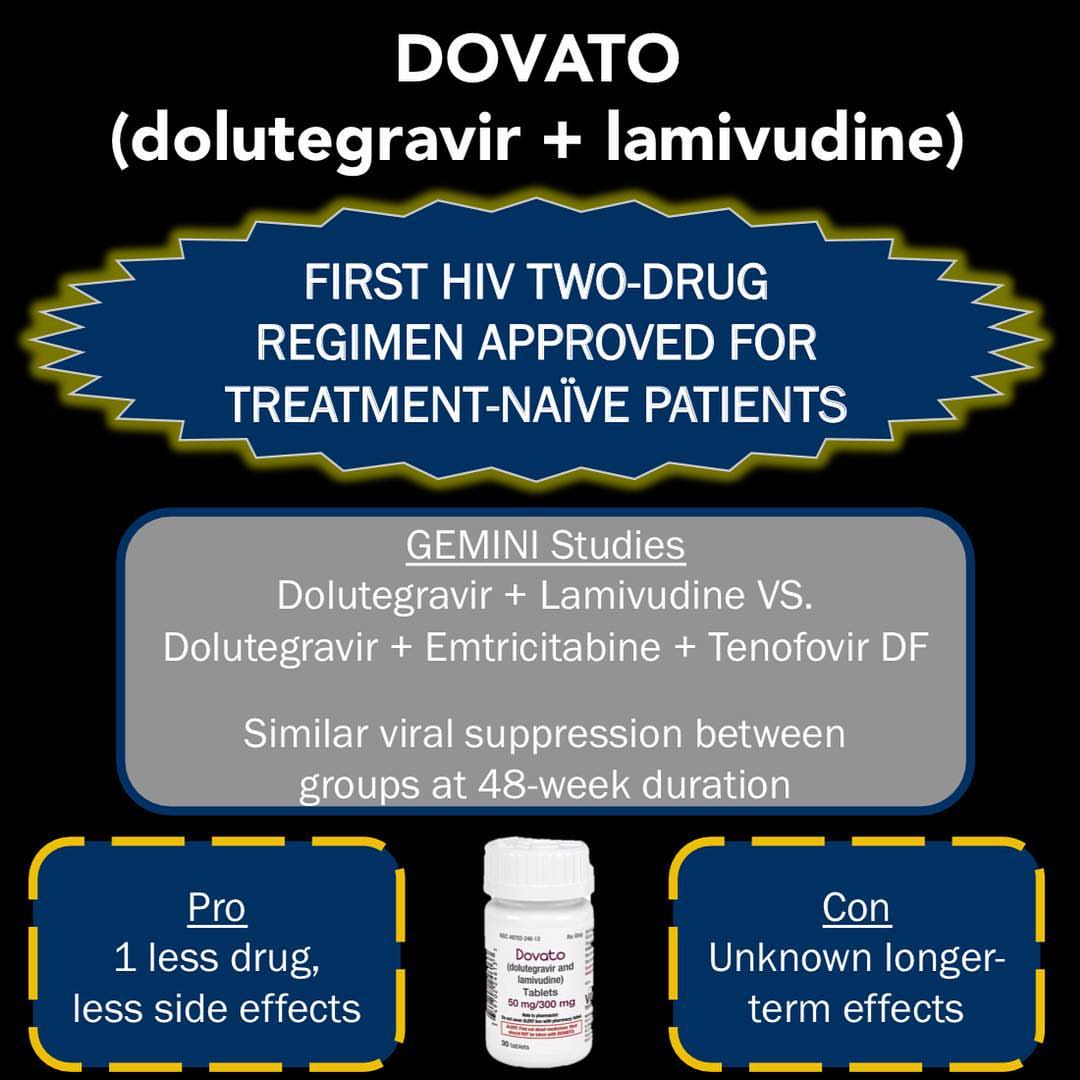
HIV/AIDS Cure 2019: approval of drugs
The Food and Drug Administration approved two drugs this month. The drugs are of the regime known as Dovato. The two drugs have a combination of chemicals.
These are of Dolutegravir and Lamivudine. The two are for prescription as HIV drugs. This, however, is mixed with other treatments as well.
The drug Dovato is particularly for HIV patients who don’t have a history. This means that the patients who have not received the anti-retroviral drugs will be the given Dovato.
It is one a kind drug. This is because it is a two-drug and has a fixed dose. Moreover, it also has a fixed regime. Therefore it is the first of this kind to get approvals of FDA.
The approval of this drug comes as progress. This progress is towards the cure of HIV/AIDS. The drug, therefore, will give HIV patients a better life. Moreover, it will also guarantee a healthier survival.
Adverse effects of the drug
The drug has been tested on several patients. According to the FDA, 1,433 HIV patients tried and tested this drug. These patients don’t have a history of anti-retroviral drugs. Moreover, clinical trials were controlled.
The drug has adverse effects, according to the FDA. These are nausea, fatigue, and insomnia. Moreover, the drug also causes diarrhea and headache.
The HIV patient will have to take one tablet of Dovato. This will be on a daily basis. However, pregnant women must not take this medicine. This is especially during their first trimester.
Moreover, patients with Hepatitis B must take Dovato along with other prescriptions. They can also try other regimes.
For more updates on cures to various diseases as well as health guides, please stay tuned to Hiptoro.



